Details of the Drug
General Information of Drug (ID: DMRMFP4)
| Drug Name |
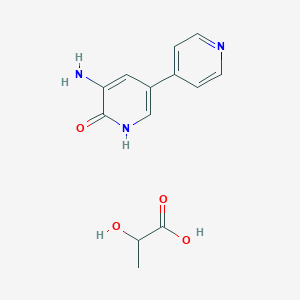
Inamrinone Lactate
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Amrinone; inamrinone; 60719-84-8; Wincoram; Inocor; Cordemcura; Cartonic; 5-Amino-[3,4'-bipyridin]-6(1H)-one; Amrinonum [INN-Latin]; Amrinonum; Amrinona; Amrinona [INN-Spanish]; 3-Amino-5-(4-pyridinyl)-2(1H)-pyridinone; Win-40680; Amcoral; 5-Amino-(3,4'-bipyridin)-6(1H)-one; WIN 40680; AWD 08-250; 3-amino-5-pyridin-4-yl-1H-pyridin-2-one; UNII-JUT23379TN; CCRIS 3794; Amrinone lactate; MLS000069829; EINECS 262-390-0; 5-amino-3,4'-bipyridin-6(1H)-one; BRN 0744819; 5-Amino(3,4'-bipyridin)-6(1H)-one; SMR000058850; CHEMBL12856; JUT23379TN; Amrinone Lactate
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Cardiovascular Agents
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 277.28 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||||||
| Rotatable Bond Count | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count | 4 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 6 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
